Abstract
Background: Diffuse large B cell lymphoma (DLBCL) is the most common histologic subtype of aggressive non-Hodgkin lymphoma. Double expressor lymphoma, occurring in around 30-40% of cases of DLBCL, is associated with poor clinical outcomes (Devi K, et al. Leuk Res Rep. 2021). Although double expressor lymphomas respond poorly to standard R-CHOP therapy with an average OS of 5 months to 2 years, few treatments have proven more effective than R-CHOP at improving survival (Yetisir AE, et al. Medical Science and Discovery. 2020). Patients who have failed first-line treatment have poor prognosis and overall survival. Studies have shown that R-CHOP plus BTK inhibitors significantly prolong survival in patients with double expressor lymphomas. Zanubrutinib, a novel BTK inhibitor, has shown promising outcomes in newly diagnosed and relapsed/refractory B-cell lymphoma, with favorable anti-tumor activity (ORR 61%) in double expressor DLBCL patients (Yang H, et al. 2020 EHA EP1246).
Aims: This single-center, retrospective, historical control study is designed to evaluate the efficiency and safety of zanubrutinib in combination with R-CHOP in patients with double expressor DLBCL.
Methods: This study enrolled 7 previously untreated patients with double expressor DLBCL at our center in the period from March 2021 to April 2022. All patients were confirmed to be double expressor lymphoma by FISH and immunohistochemistry. Patients with double-hit lymphoma were excluded. Patients received rituximab 375mg/m2 intravenously at Day0, cyclophosphamide 750mg/m2 intravenously at Day1, doxorubicin 50mg/m2 intravenously at Day1, vincristine 1.4mg/m2 intravenously at Day1, prednisone 100mg/day orally at Day1-5, zanubrutinib 160mg orally bid. All patients underwent interim evaluation after cycle 2. Unfit or elderly (>72 years) patients received ZR-miniCHOP. Historical control group enrolled double expressor DLBCL patients treated with R-CHOP (R-miniCHOP for unfit or elderly patients) at our center in the period from July 2019 to April 2022. The study was approved by our institutional ethics committee.
Results:
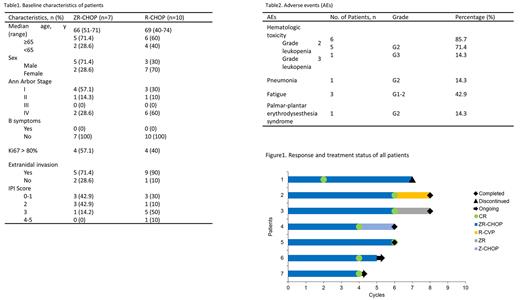
Among 7 patients treated with ZR-CHOP, the median age was 66 years (range 51-71), 5 patients (71.4%) aged≥65 years, 5 patients were males, 2 patients were females, 1 patient (14.3%) had IPI≥3 score, 2 patients (28.6%) had stage III/IV stage disease, 5 patients (71.4%) had extranidal invasion and no patient had bone marrow invasion (Table 1). The data cut-off on July 1st 2022, all patients completed 4-6 cycles of ZR-CHOP and were evaluable for efficiency. The overall response rate (ORR) of 7 patients was 100%(7/7), 5 patients (71.4%) achieved complete molecular response (CMR), 2 patient (28.6%) achieved complete response (CR) at interim evaluation (Figure1). The median follow-up time was 7 months (4-16 months). All patients survived with no disease progression. Median OS and PFS were not reached. The most common adverse events (AEs) were hematologic toxicity and fatigue. Any grade and grade≥3 hematologic toxicity were observed in 85.7% and 14.3% patients, respectively. 6 patients developed grade≥2 leukopenia and 1 patient developed pneumonia during treatment. 1 patient had palmar-plantar erythrodysesthesia syndrome. No atrial fibrillation or fatal AE occurred (Table2).
Historical control group enrolled 10 patients treated with standard R-CHOP. Baseline characteristics are comparable with ZR-CHOP arm and summarized in Table1. All patients completed 3-6 cycles of R-CHOP. ORR was 80% (8/10) with a CMR rate of 40% (4/10). The median follow-up time was 19 months (3-32 months). Disease progression occurred in 5 patients (50%). 3 patients died due to disease progression.
Conclusion: Zanubrutinib+R-CHOP (ZR-CHOP) is tolerated and effective for previously untreated patients with double expressor DLBCL. Compared with the historical R-CHOP arm in the same center, adding zanubrutinib to standard first-line therapy could potentially achieve higher ORR and CR. ZR-CHOP should be a new option for patients with double expressor DLBCL. Further phase III study is warranted.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal